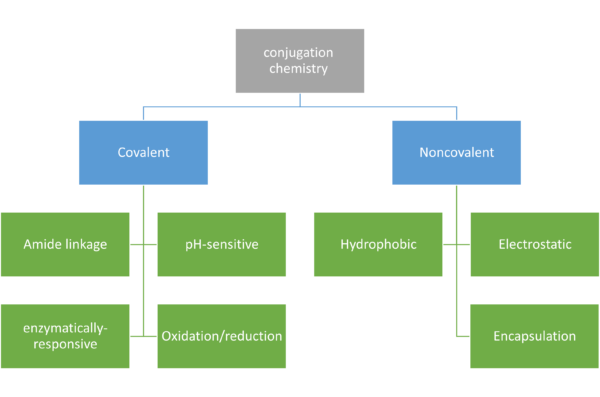
توضیحات
Abstract
When assessing novel SPION formulations, caution should be exercised since they may interact with blood-borne enzymatic cascades such as the coagulation pathways, complement system, and kallikrein-kinin system (KKS). Before inflammatory cells arrive, a process known as opsonization, which occurs quickly when a foreign substance enters a host, causes the foreign substance to collect a coating of host proteins. Adsorbed fibrinogen, which is necessary for phagocyte accumulation, can be detected as a fibrin clot by phagocytes, which causes the phagocytes to launch reactions intended to start wound healing at a site of vascular damage. High-molecular-weight kininogen and factor XII of the clotting cascade may displace adsorbable fibrinogen, which is the cause of platelet adhesion. Additionally, alterations in antibody binding or epitope exposure brought on by the unfolding of adsorbed fibrinogen during opsonization may promote additional coagulation and inflammation. By encouraging thrombosis or bleeding, inflammatory reactions brought on by SPIONs might change the hemocoagulation balance. Tissue factor (TF) is expressed intravascularly on monocytes during an inflammatory response, and proinflammatory cytokines activate endothelial cells. Then, activated TF and activated FVII may combine to activate FX and FIX. The activated partial thromboplastin time (APTT) is changed by the kallikrein-kinin system, which also helps to activate the intrinsic coagulation pathway. Glass, kaolin, and celite are examples of negatively charged materials that can activate FXII by stimulating the KKS. Prekallikrein is subsequently converted to kallikrein by activated FXII through a positive feedback loop. Clinical signs of this include hypotension, erythema, hyperfibrinolysis, and the induction of a complicated inflammatory response. When plasma prekallikrein and high-molecular-weight kininogen (HK) attach to the iron oxide core of dextran-coated SPIONs, the kallikrein-kinin system may be activated. As a result, dextran-coated SPIONs should be examined with caution. IL1β levels were previously demonstrated to increase in response to polyvinyl alcohol (PVA) coated SPIONs, but this impact was reduced by removing free-floating PVA and lowering the zeta potential of the coated SPIONs.
There was evidence of greater interaction between blood and PAA-coated SPIONs when compared to hyaluronic acid (HA), chitosan (CS), and other coatings for SPIONs. RBC hemolysis rates were 23% when this was present, compared to 7.5% for HA and 5.1% for CS. When compared to the HA and CS coatings, the PAA-coated SPIONs also showed the greatest level of RBC membrane deterioration, aggregated platelets, and noticeably extended prothrombin, activated partial thromboplastin, and thrombin times. The numerous -OH and -COOH groups of PAA were hypothesized to be responsible for the rise in activated C3 of the classical pathway of the complement system, whereas the delayed coagulation time was caused by the loss of one or more coagulation components.
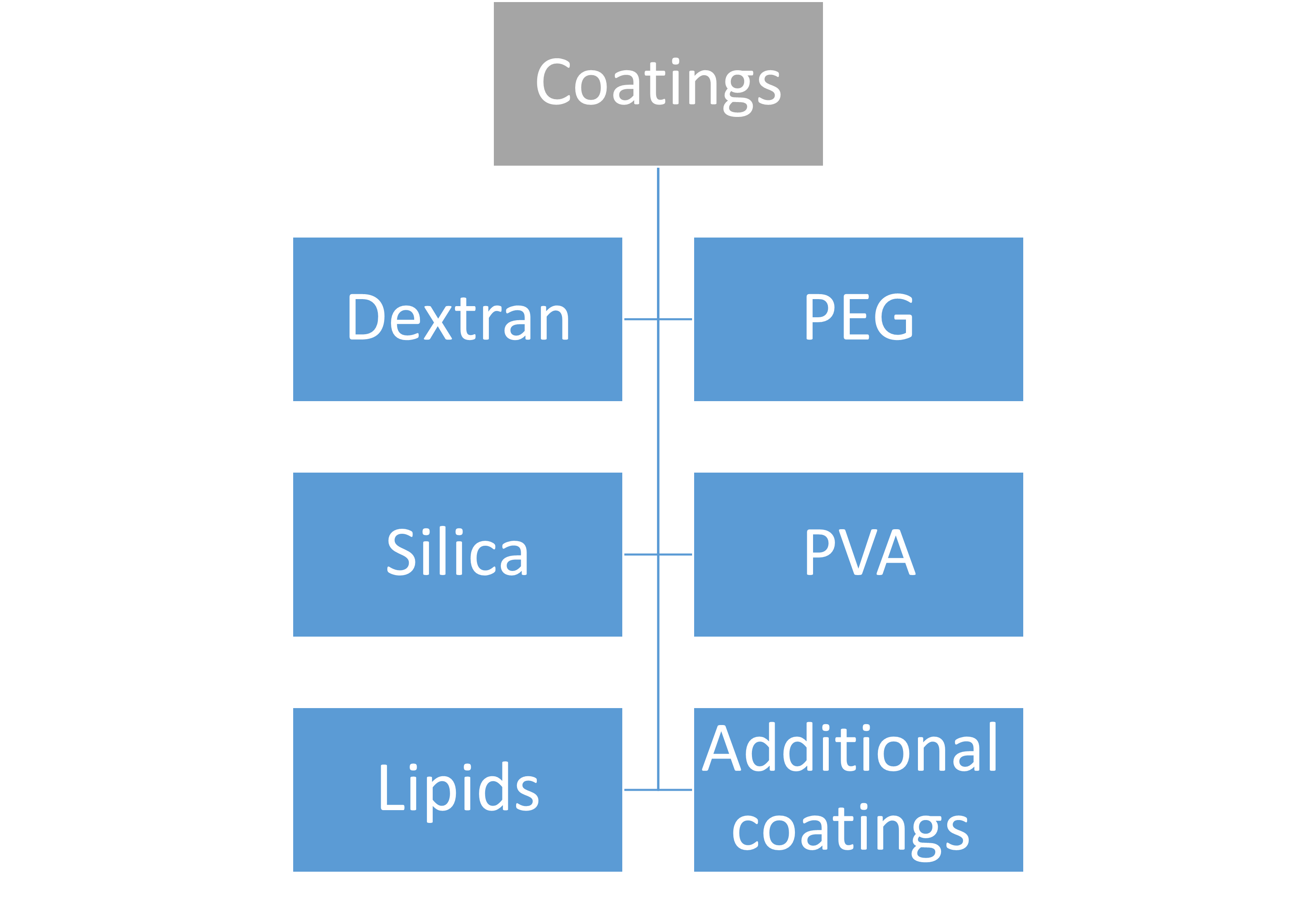
Contrary to polyvinylpyrrolidone and uncoated IONPs, PEG coating of IONPs demonstrated enhanced adsorption and activation of the complement system with elevated concentrations of the C3a, C4a, C5a, C5b-9, IL-1B, IL-6, and TNF-α. Anaphylatoxins are the soluble byproducts of complement activation C3a, C4a, and C5. They function by causing localized or systemic inflammation. To create a hole in the cell membrane that allows free molecule flow into and out of the cell, C5b-9 binds to and polymerizes several copies of C9. This causes cell death. These studies collectively highlight the significance of the coating when assessing the security and biocompatibility of SPIONs. Although at concentrations below 100 mg/mL, IONPs of tiny size exhibit very little to no cytotoxicity, Aggregates may have a different behavior as a result of the larger particle size. With concentrations of 3 mg/mL for SPIONs 16 nm in size, uncoated SPIONs exhibit a strong propensity to aggregate or precipitate in saline, a lot of effort has gone into creating coverings for SPIONs to stop this precipitation and aggregation while avoiding cytotoxicity. These coatings might include PEG and other compounds such as dextran, chitosan, as well as other polymers. The PEG, PVA, and dextran high molecular weight coatings hinder SPION aggregation by interfering with the potent magnetic dipole–dipole interactions between the iron oxide cores. Increasing relaxation durations for improved imaging outcomes is another benefit of encasing SPIONs in coatings and inhibiting or postponing the release of Fe2+ when in contact with lysosomes. The ideal biocompatible coating should be able to break down into particles smaller than 5 nm to be excreted by the kidneys with the least amount of cytotoxicity. The efficacy of cell labeling can also be improved by changing the surface coating of the SPION to have a cationic surface as opposed to negatively charged or neutral surface coatings. Topics of this chapter are presented hierarchically in the below figure.
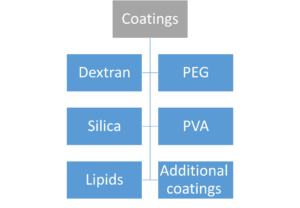
Coatings

When assessing novel SPION formulations, caution should be exercised since they may interact with blood-borne enzymatic cascades such as the coagulation pathways, complement system, and kallikrein-kinin system (KKS). Before inflammatory cells arrive, a process known as opsonization, which occurs quickly when a foreign substance enters a host, causes the foreign substance to collect a coating of host proteins. Adsorbed fibrinogen, which is necessary for phagocyte accumulation, can be detected as a fibrin clot by phagocytes, which causes the phagocytes to launch reactions intended to start wound healing at a site of vascular damage. High-molecular-weight kininogen and factor XII of the clotting cascade may displace adsorbable fibrinogen, which is the cause of platelet adhesion. Additionally, alterations in antibody binding or epitope exposure brought on by the unfolding of adsorbed fibrinogen during opsonization may promote additional coagulation and inflammation. By encouraging thrombosis or bleeding, inflammatory reactions brought on by SPIONs might change the hemocoagulation balance. Tissue factor (TF) is expressed intravascularly on monocytes during an inflammatory response, and proinflammatory cytokines activate endothelial cells. Then, activated TF and activated FVII may combine to activate FX and FIX. The activated partial thromboplastin time (APTT) is changed by the kallikrein-kinin system, which also helps to activate the intrinsic coagulation pathway. Glass, kaolin, and celite are examples of negatively charged materials that can activate FXII by stimulating the KKS. Prekallikrein is subsequently converted to kallikrein by activated FXII through a positive feedback loop. Clinical signs of this include hypotension, erythema, hyperfibrinolysis, and the induction of a complicated inflammatory response. When plasma prekallikrein and high-molecular-weight kininogen (HK) attach to the iron oxide core of dextran-coated SPIONs, the kallikrein-kinin system may be activated. As a result, dextran-coated SPIONs should be examined with caution. IL1β levels were previously demonstrated to increase in response to polyvinyl alcohol (PVA) coated SPIONs, but this impact was reduced by removing free-floating PVA and lowering the zeta potential of the coated SPIONs.
URL: https://link.springer.com/chapter/10.1007/978-981-99-6507-6_12
نویسنده: Mohammad-Nabil Savari
5